
Patient and Public Acceptance of Digital Technologies in Health Care: Protocol for a Discrete Choice Experiment (English)
Background, objectives, and methods
An E-BRAiN newsletter issued by Ann-Kathrin Fischer and Axel Mühlbacher
Background: The value of digital neurorehabilitation
Strokes challenge healthcare systems uniquely. While age-adjusted stroke-related deaths have decreased, new stroke cases have increased. Strokes cause lasting disabilities; neurorehabilitation aims to reduce these. With rising demand and fewer specialists, innovative solutions are vital.
The digital shift offers ways to enhance healthcare through technology. The E-BRAiN project explored a humanoid robot for therapy support. Patient acceptance of innovations is crucial for informed decisions. Acceptance entails evaluating pros and cons and resulting behavior. It's achieved when positive attitudes and usage outweigh negatives.
Method of the study: conducting a discrete choice experiment
Study objectives
Discrete choice experiments help identify preference-based trade-offs, influencing acceptance factors. Therefore, a discrete choice experiment was developed to explore how digital technology criteria affect patient and public acceptance. It investigated patient preferences for analyzing the value of digital interventions. Preferences involve observed behaviors guiding decisions between health options. This research assessed therapy preferences based on specific attributes.
Study population
A web-based questionnaire was developed for two study populations. The experimental group comprise patients diagnosed with a stroke, while the control group includes individuals from the general population. Only individuals aged 18 and above, residing in Germany, and capable of reading and understanding German were included in both groups.
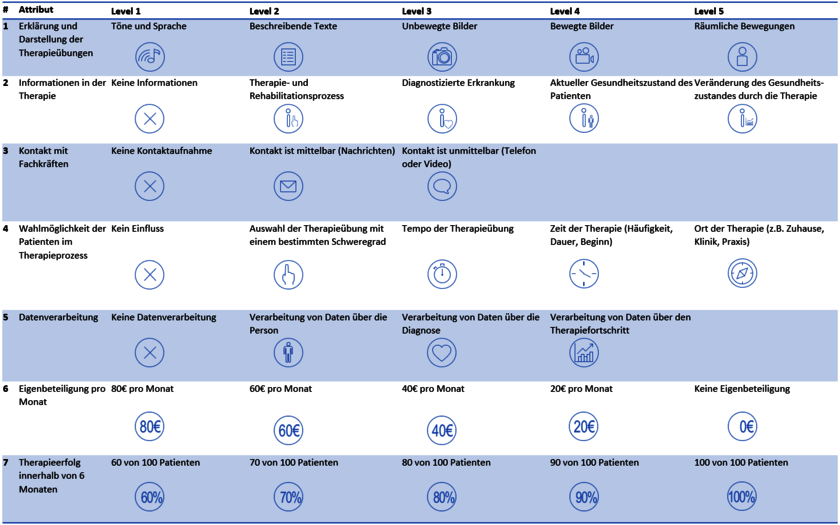
Table 1 – Attributes and Levels
Choice tasks
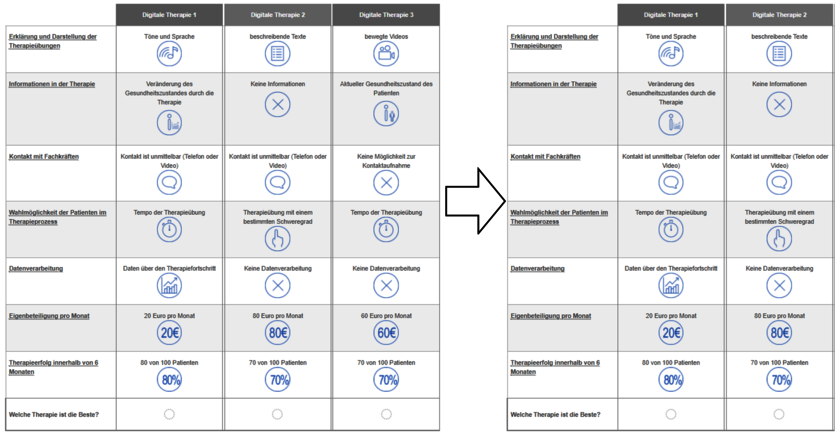
Seven attributes were used to create choice tasks for a web-based questionnaire. These tasks involved comparing therapy options and choosing the preferred one. Initially, three options were presented based on the attributes, and after selecting the best among them, the remaining two were shown. From these, the best and second-best options were chosen in six tasks (see Figure 1: Choice task (a) best alternative; (b) second best alternative).
Experimental design
The web questionnaire was based on an underlying experimental design. The experimental design is a fractional-factorial efficient Bayesian design (D-error). This design pertains to a statistical approach aimed at providing efficient and accurate estimations. The focus of the design is to achieve an optimal balance among the given attributes and levels (e.g., frequency of attributes and levels across the six choice tasks across the total number of respondents).
Final survey design
The final study design includes a web-based questionnaire with choice tasks, background questions, and cognitive tests. Stroke patients often face cognitive impairments, necessitating careful attention to the survey layout. Specific attention was given to stroke patients' cognitive impairments, ensuring a clear and manageable layout. Symbols were designed for visual clarity. The survey duration was limited to 30 minutes for better data quality.
Quality assurance
The questionnaire underwent pretest interviews with stroke patients and the general population. After collecting data from 150 control group participants in the web-based survey, it was evaluated and refined. The analysis also checked for unrealistic response times and non-rational patterns, ensuring answer quality and statistical significance.
Data analysis
The results will be computed using a conditional logit model and a mixed logit model. These models allow for identifying differences in variables (attributes and attribute levels) and making predictions about the choice probability of therapy options. Furthermore, heterogeneity will be analyzed through latent class analyses, and heteroskedasticity will be examined using heteroskedastic models. Thus, variations based on respondents' characteristics will be investigated.
Publishing results
The literature review, qualitative preliminary study, survey development, and pilot testing have been completed. Recruitment of the experimental and control groups started in January 2022. Data collection and analysis will be finalized in the last quarter of 2023. The study protocol for this research was published in July 2023.
Outlook and interpretation of the study
This study will provide information on patient preferences to analyze the criteria influencing acceptance and value of innovative interventions using digital technologies. Researchers, developers, healthcare providers, and decision-makers often face tough choices regarding development, reimbursement, and intervention selection. These decisions require data on the value of clinical and non-clinical criteria. Since patients ultimately experience the positive and negative therapy attributes, decisions should align with and reflect patients' values.
Figure 1 - Choice task (a) best alternative; (b) second best alternative
Reference: Ann-Kathrin Fischer, Axel Mühlbacher. Patient and Public Acceptance of Digital Technologies in Health Care: Protocol for a Discrete Choice Experiment. JMIR Res Protoc (forthcoming). doi:10.2196/46056
Acknowledgement
The authors of the study would like to thank Prof. Dr. Thomas Platz and Prof. Dr. Thomas Kohlmann for their valuable discussions and constructive support. Additionally, the authors extend their gratitude to Stefanie Tobschall and Stephanie Bobe for their assistance in planning numerous project steps. Special thanks go to Ann Louise Pedersen for her support, guidance, and organization of patient surveys.
This study is part of the research project E-BRAiN, funded by the European Social Fund (Reference: ESF/14-BM-A55-0001/19-A02) and the Ministry of Education, Science, and Culture of the State of Mecklenburg-Western Pomerania, Germany.
Ein Forschungsverbund mit Beteiligung der Universität und Universitätsmedizin Greifswald, Universität Rostock und Hochschule Neubrandenburg
Verbund-Koordinator Prof. Dr. med. Thomas Platz | Ansprechpartner Team der AG Neurorehabilitation |
Impressum | Datenschutz | Newsletter abmelden
ebrain-science.de
